
Fluorophores
Fluorophore Types
There are several types of fluorescent compounds with very different chemical composition. Accordingly, they have specific advantages and disadvantages which need to be considered when choosing and optimizing them for a fluorescence application.
Small organic fluorophores
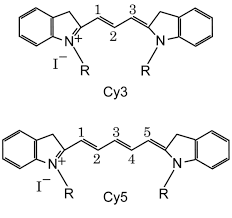
These are small organic molecules which are currently the most popular fluorophores in biological applications. Popular examples are Cy3, Cy5, FITC and Alexa dyes.
Fluorescent proteins
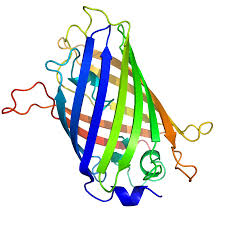
These kind of fluorophores were originally found in jellyfish and provide the unique advantage that they can be genetically engineered into live cells as fusion proteins. This way, specific structures can be labelled and observed within intact cells or even organisms. Popular examples are GFP or phycoerythrin.
Quantum dots
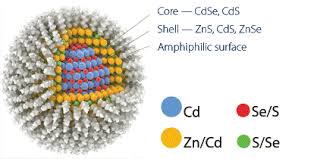
These are nanometer sized clusters of semiconductor atoms. Due to unique quantum effects, quantum dots can be excited in a very wide wavelength range and the emission wavelength can be engineered specifically. Due to the toxic components, most commercially available quantum dots are contained in a shell. They are furthermore coated with organic molecules to obtain certain surface characteristics.
Inorganic fluorophores (e.g. Eu3+)
The term “fluorescence” itself is based on the effects first observed in a mineral called Fluorite (CaF2). Later it was found out that Fluorite itself is not fluorescent, but that impurities of divalent Europium caused the observed fluorescence. Europium fluorescence shows a very large stokes shift and is extremely photostable. It is therefore used at large scale for TV screens. However, it can also be used in diagnostic applications.
Fluorophore Interactions
For more complex applications, there is a multitude of interactions between fluorophores and their environment which can be used for optimization.
- Fluorophores can react to salt concentration and pH
- Fluorophores can transfer their energy to other fluorophores in close proximity, thus enabling interaction analysis of proteins/enzymes
- Fluorophores can be quenched by certain molecules
- Fluorophores can be optimized by addition of reducing or oxidizing agents
contact
GATTAquant GmbH
Staffelseestraße 8
DE-81477 München
Phone: +49 89 2153 720 80
INFO@GATTAQUANT.COM
funded by

Payment Options


 English
English German
German Chinese
Chinese